By retarding either the anodic or cathodic reactions the rate of corrosion can be reduced. This can be achieved in several ways :
4.3.1 Conditioning the Metal
This can be sub-divided into two main groups:
(a) Coating the metal, in order to interpose a corrosion resistant coating between metal and environment. The coating may consist of:
(i) another metal , e.g. zinc or tin coatings on steel,
(ii) a protective coating derived from the metal itself,
e.g. aluminium oxide on “anodised” aluminium,
(iii) organic coatings, such as resins, plastics, paints, enamel, oils and greases.
The action of protective coatings is often more complex than simply providing a barrier between metal and environment. Paints may contain a corrosion inhibitor
ALSO……
Underbody structural components are typically coated to provide a first line of defense against corrosion. For light truck frames, the two most common coatings are hot melt wax and electrocoat (E-coat). Paints are also used on current light truck frames. Conversion coatings enhance the adhesion of electrocoat or paint, and they are commonly used in conjunction with these two coating types. Many underbody structural components, such as front rails on passenger cars, are made from sheet steel pre-coated with a metallic coating, e.g., galvanized or galvanneal sheet steel. Autophoretic and powder coatings are also used on underbody structural components.
*Coating type : 1. Internal Lining

Description:
Internal coating using a two component liquid epoxy based paint.
Features:
This coating system has excellent anti-friction properties and good resistance to chemicals.
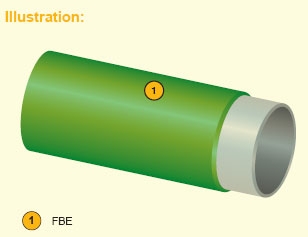
Stand alone coating system.
Features:
This coating system has adequate mechanical properties and effective anti-corrosion properties with resistance to high temperature operating service up to 120°C depending on raw materials used.
3. Dual Fusion Bonded Epoxy (D-FBE ) coating
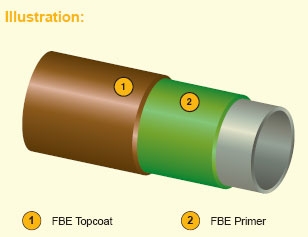
Fig (4-3) Dual Fusion Bonded Epoxy (D-FBE ) coating
2-layer coating system composed of FBE primer (first layer), FBE topcoat (top layer).
Features:
This coating system has good mechanical properties and effective anti-corrosion properties and resistance to high temperature operating service up to 110°C or 150°C depending on raw materials used.
4. Bitumen / Asphalt Enameln (AE) Coating

Description:
Multi-layer coating system composed of synthetic primer (first layer), enamel / inner wrap / enamel layer(s) (second and, if any following layers) and outer wrap layer (top layer).
Features:
This coating system has adequate mechanical properties and effective anti-corrosion properties with resistance to temperature operating service up to 90°C.

Fig (4-5) Three Layer Polypropylene (3LPP) Coating
3-layer coating system composed of FBE primer (first layer), polypropylene based adhesive copolymer (second layer) and polypropylene based topcoat (top layer).
Features:
This coating system combines excellent mechanical properties and resistance to high temperature operating service up to 110°C or 150°C depending on raw materials used.
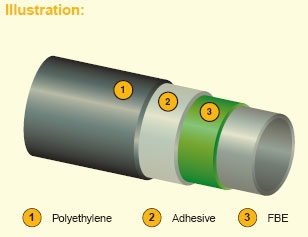
3-layer coating system composed of FBE primer (first layer), polyethylene based adhesive copolymer (second layer) and polyethylene based topcoat (top layer).
Features:
This coating system combines excellent mechanical properties and resistance to temperature operating service up to 60°C (LDPE & MDPE) or 80°C (HDPE) depending on raw materials used.
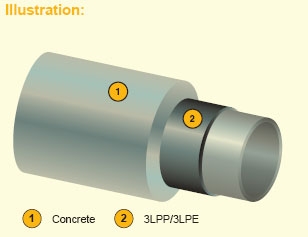
Fig (4-7) Concrete Weight Coating (CWC)
Description:
Weight coating system composed of cement, water, aggregates, heavy or light depending on the required density, and reinforcement.
Features:
Concrete weight coating is used to provide pipe stability on the sea bed as well as superior mechanical protection. It can be manufactured in a range of densities to suit the project specification.
8. Polyurethane Insulation Coating
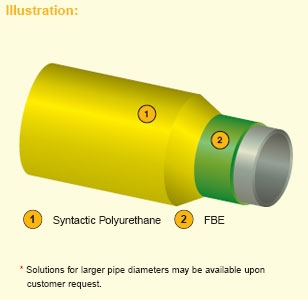
Fig (4-8) Polyurethane Insulation Coating
2-layer coating system composed of FBE and syntactic polyurethane. Polymer or glass microspheres are blended to provide excellent thermal insulation properties.
Features:
Polyurethane insulation systems are designed to cover various water depths. Shallow water products (SPU) are based on a PU matrix into which polymer microspheres are blended to provide excellent thermal insulation properties. Deepwater applications (DWPU) are addressed using a range of products into which glass microspheres are blended. Both products have extensive track records.

Fig (4-9) Polypropylene Insulation Coating
Multi-layer coating system composed of polypropylene outer shield, solid polypropylene and foamed polypropylene / syntactic polypropylene.
Features:
Solid polypropylene is used as both anti-corrosion coating and thermal insulation coating where the thermal requirements are not too demanding. Another coating system is side extruded polypropylene foam which is used for thermal insulation of pipelines for water depths up to 600m. Syntactic polypropylene is used to achieve a balance between good thermal performance and thermal insulation capability for deepwater applications.
to produce a more corrosion resistant alloy, e.g. stainless steel, in which ordinary steel is alloyed with chromium and nickel. Stainless steel is protected by an invisibly thin, naturally formed film of chromium oxide Cr2O3
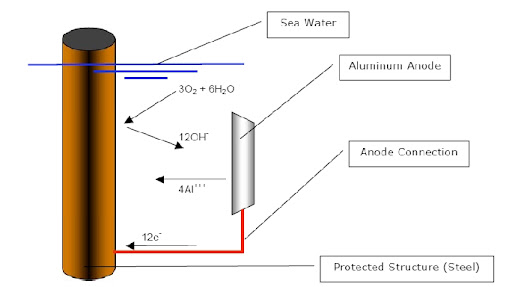
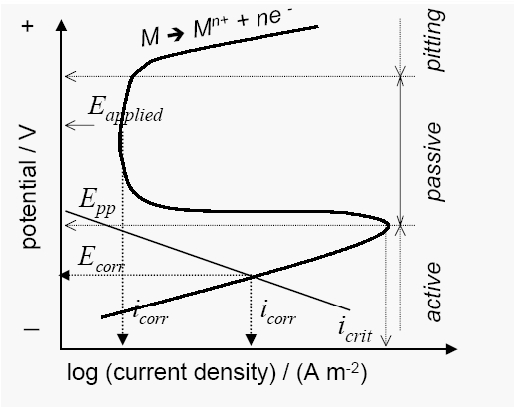
4.3.2 Conditioning the Corrosive Environment






0 التعليقات:
Post a Comment